Testing of medical packaging
Excerpt from the guideline DIN CEN ISO_TS 16775
Sterile barrier systems are designed to enable sterilization, provide physical protection, maintain the sterility of their contents to the point of use and enable aseptic delivery. Depending on the handling, transportation or storage conditions, a sterile barrier system can be combined with additional protective packaging to create a packaging system. ISO 11607-1 specifies requirements for materials, sterile barrier systems and packaging systems, including the validation of the design of a packaging system; ISO 11607-2, on the other hand, specifies requirements for the validation of packaging processes. The requirements set out in these standards are generic and apply to healthcare facilities and wherever medical devices are packaged and sterilized.
ENVIRONMENTAL SIMULATION / AGEING (shelf life)
Environmental simulation includes test methods for investigating external environmental influences on packaging. The packaging is exposed to an aging process in constant heat in accelerated or real time and the effects of the environment with regard to performance, functional behavior and service life are determined.

- Accelerated ageing (ASTM F1980)
- Real-time aging (ASTM F1980)
TRANSPORTSIMULATION (performance test/qualification)
Transport simulation includes test methods for examining external transport conditions on packaging. The packaging is subjected to vibration, impact and compression tests, tilt and drop tests and tensile strength tests to determine the effects of various freight, storage and transportation conditions.


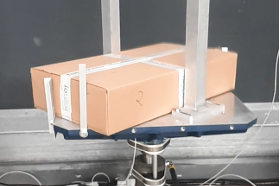

- Transport simulation (ASTM D4169)
PACKAGING/STERILE GOODS PACKAGING
The primary objective of packaging systems for medical devices that are sterilized in the final packaging is to maintain sterility until use and for aseptic delivery to the patient (DIN EN ISO 11607-1). For this purpose, appropriate tests must be carried out, which ultimately enable the development and specification of packaging systems and medical devices.
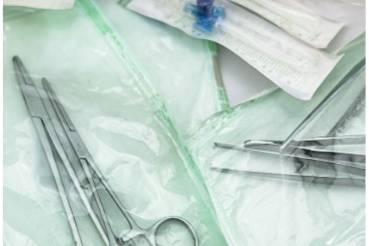
Test methods in packaging testing
Checking the integrity of the sterile barrier system:
- Visual inspection (ASTM F1886 / F1886M)


- Bubble test (ASTM F2096)

- Testing for impermeability and continuity of the seal (ASTM F1929, ASTM F3039)

- Testing for fine holes in the plastic composite film (DIN EN 868, Part 5)

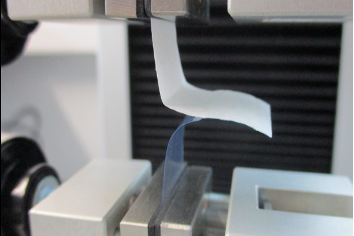
Windability:
- Determination of peel characteristics (peelability test DIN EN 868, part 5)
- Determination of the seal seam width (DIN EN 868, part 5)

Determination of the seal strength:
- Tensile strength test of sealing seams (DIN EN 868, Part 5, ASTM F88)
Test for germ tightness:
- Germ-tightness test (DIN 58953-6)
- With moisture:

- With air passage:

- Microbial evaluation of porous packaging materials (ASTM F1608)
